CAR-T Cell Therapy: Revolutionizing Cancer Treatment with Innovation and Accessibility
Cancer treatment has entered a new era with CAR-T cell therapy, a cutting-edge form of immunotherapy that is transforming outcomes for patients with certain cancers. In this comprehensive overview, we explain what CAR-T therapy is, how it works, who can benefit, and how new programs – including an initiative by Burjeel Hospital, Abu Dhabi in the UAE – are making this life-saving treatment more accessible.
What Is CAR-T Cell Therapy?
CAR-T stands for Chimeric Antigen Receptor T-cell therapy. It is a specialized treatment that uses a patient’s own immune cells (T cells) to fight cancer. CAR-T therapy is considered a type of cell-based gene therapy, because the patient’s T cells are genetically modified in a lab to help them recognize and destroy cancer cells. This approach essentially turns the patient’s immune cells into “living drugs” that continue to multiply and combat cancer over time.
CAR-T cell therapy has shown remarkable success in treating certain blood cancers – especially leukemias and lymphomas – that were not responding to traditional treatments. In some cases, CAR-T therapy can eliminate all signs of cancer (complete remission), even when other therapies have failed. Because of these results, CAR-T has generated hope as a potential cure or long-term control for otherwise aggressive cancers.
However, CAR-T therapy is not a first-line treatment and is usually considered when standard treatments (like chemotherapy or stem cell transplant) haven’t worked or when the cancer has come back (relapsed). It is currently approved mainly for certain blood cancers such as: acute lymphoblastic leukemia (ALL), diffuse large B-cell lymphoma (DLBCL) and some other non-Hodgkin lymphomas, mantle cell lymphoma, follicular lymphoma, and multiple myeloma. Research is ongoing to expand CAR-T therapy to other cancers, including some solid tumors, but most successes so far have been in blood cancers.
How CAR-T Cell Therapy Works
CAR-T therapy is a multi-step process that modifies a patient’s T cells to attack cancer. Here’s an overview of how it works:
- T-Cell Collection: Doctors first collect T cells from the patient’s bloodstream. This is done via a procedure called leukapheresis, which separates white blood cells (including T cells) from the blood and returns the rest of the blood to the body. The collection process is done in a specialized center and can take a few hours.
- Genetic Modification: The T cells are then sent to a laboratory where they are genetically engineered. Scientists insert a new gene into these T cells that encodes a special receptor called a chimeric antigen receptor (CAR). This CAR is like a custom GPS system that equips the T cells to recognize a specific protein (antigen) on the cancer cells. For example, many leukemia and lymphoma cells have an antigen called CD19, so a CAR targeting CD19 can direct T cells to those cancer cells.
- Cell Expansion: The modified T cells (now “CAR-T cells”) are multiplied in the lab. Over a couple of weeks, the lab grows hundreds of millions of these CAR-T cells. This expansion ensures there are enough cells to mount an attack on the cancer when returned to the patient. During this time, the patient might receive chemotherapy (sometimes called lymphodepleting chemo) to make space in the immune system for the incoming CAR-T cells.
- Infusion of CAR-T Cells: After expansion, the CAR-T cells are infused back into the patient’s bloodstream, similar to a blood transfusion. Once infused, these cells travel through the body to seek out and bind to cancer cells that have the target antigen. The CAR on the T cell locks onto the cancer cell, activating the T cell to kill the cancer. The engineered T cells not only destroy tumor cells, but also continue to live and reproduce in the body for some time, providing ongoing surveillance against the cancer.
- Cancer Cell Destruction: By recognizing the cancer’s specific “signature” (antigen), CAR-T cells can attack tumors with precision. They act as a “living drug” that multiplies and persists, potentially giving long-term protection. In some patients, CAR-T cells have remained in the body for months or even years, keeping the cancer in remission.
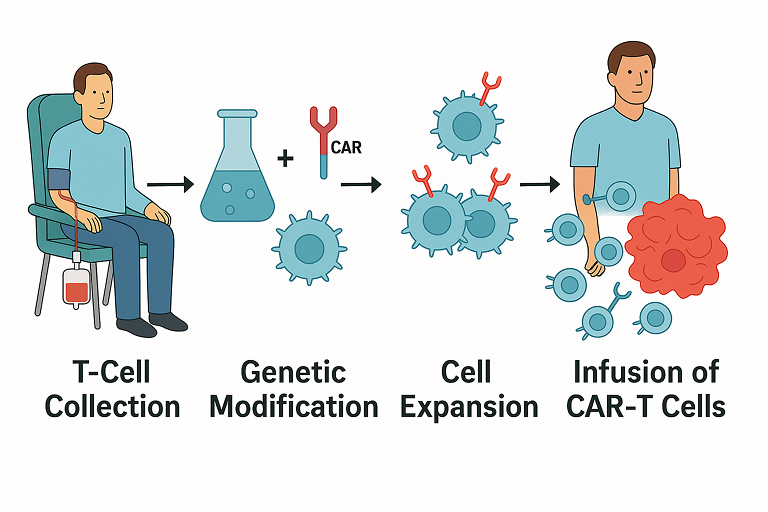
Figure: Overview of how CAR T-cell therapy is administered. First, T-cells are collected from the patient’s blood in a process called leukapheresis. Next, in the laboratory these T-cells are genetically modified to express a chimeric antigen receptor (CAR) that targets a specific cancer antigen, and then the cells are expanded (multiplied). Finally, the engineered CAR-T cells are infused back into the patient, where they seek out and destroy cancer cells.
This highly personalized process means each CAR-T dose is unique to the patient. The entire procedure from T-cell collection to infusion can take a few weeks to complete. During this period, patients are closely monitored and may receive therapy to control the cancer if needed while awaiting their CAR-T cells.
Benefits and Success Rates of CAR-T Therapy
CAR-T therapy has demonstrated impressive results, especially in patients who had exhausted other treatment options. Some key benefits and outcomes include:
- High Remission Rates: Clinical trials and studies have shown high initial remission rates in certain cancers. For instance, in a study of children with advanced acute lymphoblastic leukemia, over 85% achieved complete remission after CAR-T treatment. Many adult lymphoma patients who had no responses to chemotherapy have also seen their tumors shrink or disappear with CAR-T therapy.
- Potential for Long-Term Cure: CAR-T is sometimes described as a “one-time” treatment that can result in long-lasting effects. Unlike regular chemotherapy that requires continuous cycles, CAR-T is given once (or a short series of infusions) and the modified cells can remain active. In the pediatric study mentioned, about 60% of those children remained cancer-free one year after a single CAR-T infusion. In some patients, CAR-T cells have persisted and kept the cancer in check for years. This offers hope that CAR-T could be a curative therapy for a subset of patients, although not everyone will have a permanent remission.
- “Last Resort” Effectiveness: Perhaps the most defining benefit is that CAR-T therapy can work when other treatments fail. Patients with relapsed or refractory blood cancers – who might have had multiple rounds of chemotherapy, radiation, or even bone marrow transplants – have been given a new lease on life thanks to CAR-T. For these individuals, CAR-T can be truly life-saving, offering response rates far higher than any other option available for end-stage disease.
- Precision Medicine: CAR-T targets cancer cells specifically by their antigen, minimizing damage to healthy cells. It is a form of precision medicine. For example, CAR-T therapies against the CD19 antigen will seek out B-cell leukemia or lymphoma cells carrying CD19, largely sparing other cells. This is different from chemotherapy, which attacks all rapidly dividing cells (healthy and cancerous alike). That said, because CD19 is also on normal B cells, patients who receive CD19 CAR-T will temporarily lose normal B cells too, but this can be managed and is often an acceptable trade-off for eliminating cancer.
Despite these benefits, it’s important to acknowledge that CAR-T therapy doesn’t work for everyone. A significant percentage of patients may eventually see their cancer return or not respond fully to CAR-T. Researchers are actively studying why some patients do not respond or relapse, and how to improve CAR-T cell designs to make remissions more durable.
Risks and Side Effects: Why CAR-T Is Given at Specialized Centers
CAR-T cell therapy is a powerful treatment, but it can also cause serious side effects that require expert management. For this reason, CAR-T is only offered in specialized hospitals that have the necessary training and infrastructure. Two of the most significant side effects are:
- Cytokine Release Syndrome (CRS): As CAR-T cells kill cancer, they can trigger a massive immune response. Immune cells may release large amounts of cytokines (inflammatory chemicals), leading to CRS. Symptoms can include high fever, chills, drop in blood pressure, difficulty breathing, and organ dysfunction. In severe cases, CRS is life-threatening and patients may require intensive care (e.g., medications to raise blood pressure, oxygen or ventilator support). Doctors now have experience managing CRS with specific drugs (like tocilizumab, an immunosuppressant) to calm the immune reaction if it gets too severe.
- Neurological Toxicity: Some patients develop neurologic side effects after CAR-T infusion. They might experience confusion, extreme agitation, tremors, difficulty speaking, or even seizures and unconsciousness in severe cases. This condition is sometimes called ICANS (immune effector cell-associated neurotoxicity syndrome). It usually occurs within days to a couple of weeks post-treatment. While scary, most neurological effects are temporary and resolve with supportive care, but careful monitoring is essential.
- Other Side Effects: CAR-T can temporarily weaken the immune system in general. Patients often have low blood counts and are at risk of infections, so they are watched closely for fevers or signs of infection. Some may need transfusions or antibiotics during recovery. There can also be fatigue, nausea, or other side effects, but CRS and neurotoxicity are the hallmark challenges with CAR-T therapy.
Because of these risks, patients receiving CAR-T typically stay in the hospital for a couple of weeks and remain near the treating center for some time after infusion. Medical teams administering CAR-T undergo special training, and hospitals must meet certain certifications to offer CAR-T therapy. This ensures that if severe side effects occur, the team can recognize and treat them rapidly.
The need for specialized care is one reason why CAR-T has been available only in limited major cancer centers worldwide. It’s not a treatment that a small clinic can provide, given the complexity of manufacturing the cells and handling the side effects.
Availability and Cost Challenges of CAR-T Therapy
When CAR-T cell therapies were first approved around 2017, they represented a breakthrough in cancer care. However, they also came with staggering costs and logistical challenges. In the United States and Europe, CAR-T treatments have price tags well in excess of $350,000 for a single dose, and can even approach $1 million when including hospital care. Such costs put the therapy out of reach for many patients and healthcare systems. Moreover, each CAR-T dose is individually made, often in centralized facilities (sometimes even in another country), which means long turnaround times and complex supply chains.
Global access has been limited: Many countries, especially in the developing world or regions like the Middle East, Africa, and parts of Asia, have had little or no access to CAR-T therapy until recently. Patients in those regions who needed CAR-T would have to travel abroad (if they could afford it) or enroll in clinical trials. The situation has been compared to a “revolutionary but elusive” treatment – a true lifeline that only a few could actually grab onto.
Key barriers to wider CAR-T availability have been:
- Cost of Goods: Manufacturing CAR-T cells is expensive. It involves high-tech laboratories, viral vectors to genetically modify cells, and stringent quality controls. The materials and labor contribute to a high cost per patient.
- Infrastructure: CAR-T production requires Good Manufacturing Practice (GMP) facilities. Until now, only a few such facilities exist globally, and most are in North America, Europe, or East Asia. Shipping cells across continents is not only time-consuming but also adds risk (cells can lose viability) and cost.
- Expertise: The therapy needs trained experts – from lab scientists who handle the cells, to clinicians who manage patients through treatment and side effects. Building these expert teams and centers takes time.
- Regulatory and Approval: Each country has to approve CAR-T products or local manufacturing. This is a regulatory hurdle that some regions are still navigating, especially if they aim to produce CAR-T therapies locally rather than import from multinational pharma companies.
The good news is that efforts are underway globally to overcome these challenges. China, for example, has developed its own CAR-T products at lower costs, bringing treatment prices down to tens of thousands of dollars there. This demonstrates that more affordable CAR-T is possible with local innovation.
Now, the Middle East is also poised to make CAR-T therapy more accessible and affordable, thanks to new initiatives like the one by Burjeel Holdings in the United Arab Emirates.
CAR-T Therapy in the UAE: Burjeel’s Program for Affordable Treatment
One of the most exciting developments in expanding CAR-T access is happening in the UAE, where Burjeel Hospital, Abu Dhabi – part of Burjeel Holdings – has launched a program to manufacture CAR-T cell therapies locally. In partnership with a U.S.-based non-profit organization called Caring Cross, Burjeel is establishing a state-of-the-art GMP facility in Abu Dhabi to produce CAR-T cells at a fraction of the usual cost.
Revolutionizing Cancer Care in MENA: This collaboration, announced in April 2025 during Abu Dhabi Global Health Week, is set to revolutionize cancer care across the Middle East and North Africa (MENA) by providing CAR-T therapy at up to 90% lower cost than international prices. In practical terms, a treatment that might cost $400,000 abroad could be on the order of $40,000 under the Burjeel program – a game-changer for patients and health systems in the region. By bringing manufacturing in-country, costs for logistics and middle-men are slashed dramatically, and therapies can be delivered faster to patients in need.
How the Burjeel CAR-T Program Works: Burjeel’s initiative is focused on point-of-care production of CAR-T cells. Rather than using the traditional model where cells are shipped to a distant pharma company’s lab, the new facility in Abu Dhabi will be able to process patient cells on-site, potentially handling up to 200 patient samples simultaneously with its advanced equipment. Caring Cross, which has expertise in low-cost cell therapy production, is providing the technology, raw materials (like lentiviral vectors used to modify T cells), and training to the local teams.
By building local capabilities, this program addresses both affordability and accessibility. It reduces the dependency on importing very expensive CAR-T products and eliminates delays due to overseas manufacturing. The vision is to position the UAE as a regional hub for advanced cell and gene therapy, which will benefit not only patients in the UAE but also those from across MENA and even South Asia who could travel to Abu Dhabi for cutting-edge treatment.
Initial Focus and Future Plans: The first phase of Burjeel’s CAR-T program is concentrating on blood cancers – specifically leukemias and lymphomas, which are the diseases where CAR-T has already proven highly effective. This means patients with aggressive blood cancers in the region may soon have a local option for CAR-T therapy, dramatically improving their prognosis. Looking ahead, the partnership also intends to explore CAR-T and similar cell therapies for other serious diseases; intriguingly, there are plans to research applications for diseases like HIV in the future. This signals that the infrastructure being put in place could in time be leveraged beyond oncology, potentially contributing to cures for viral diseases or other conditions using cell-based therapies.
Impact on Patients and Healthcare: For patients, the Burjeel CAR-T program could mean life-saving treatment without the prohibitive cost. Many families in the past might have faced the heartbreaking reality that even if CAR-T could save their loved one, they simply could not afford it or access it. With local production, more patients can be treated, and much sooner after needing therapy. This program also emphasizes training local healthcare providers and building expertise, which contributes to a sustainable ecosystem. In the long run, it can drive innovation and research in the region, attracting clinical trials and talent, further enhancing treatment options available locally.
It’s worth noting that this initiative in the UAE aligns with a broader global trend: moving towards decentralized, locally-produced advanced therapies. By reducing reliance on a few manufacturing sites globally, such models hope to ensure equity in healthcare – so that a breakthrough like CAR-T isn’t just a hope for patients in wealthy countries, but a reality for patients everywhere.
The Future of CAR-T: What Patients Can Expect
CAR-T cell therapy is an evolving field. Since the first CAR-T treatments were approved in 2017, there are now six FDA-approved CAR-T therapies for various leukemias, lymphomas, and myeloma, and ongoing trials are exploring new targets and cancer types. Researchers are even working on newer generations of CAR-T cells that might work in solid tumors (like lung or brain cancers) and on making CAR-T cells “off-the-shelf” (using donor cells, so they are immediately available without the weeks-long manufacturing for each patient).
For patients and the general public, here are a few key points about what the future holds:
- More Treatment Centers Worldwide: As seen with the Burjeel program, more centers around the world will likely start offering CAR-T therapy, either by partnering to manufacture locally or by acquiring the technology. This means patients may not have to travel as far to receive CAR-T in the future.
- Lower Costs Over Time: Just as we’ve seen in the UAE with a 90% cost reduction initiative, competition and innovation are expected to drive costs down. Non-profit models (like Caring Cross) and academic centers are developing CAR-T therapies that might be cheaper than commercial products. In a few years, CAR-T might be much more affordable, hopefully covered by insurance or governments due to its proven benefits.
- Broader Disease Applications: While today CAR-T is mainly for certain blood cancers, tomorrow we might see it used for other cancers or even non-cancerous diseases. Clinical trials are underway for CAR-T in autoimmune diseases (like lupus or multiple sclerosis) and infectious diseases. The principle of genetically empowering immune cells has a wide range of potential uses.
- Patient Empowerment and Education: With highly sophisticated treatments like CAR-T, patient education is crucial. Hospitals are creating resources to help patients understand what to expect, and survivors of cancer treated with CAR-T are sharing their experiences. This builds community knowledge and comfort with the therapy. If you or a loved one has a cancer that might be treated with CAR-T, it’s important to consult with an oncologist who has expertise in this area. They can explain the eligibility, process, potential benefits, and risks in detail.
- Continued Research and Improvement: The medical community is learning more every day – for instance, how to minimize side effects like CRS, how to select the patients most likely to benefit, and how to improve CAR-T cells so they last longer in the body. Over 1,000 CAR-T clinical trials are ongoing worldwide, underscoring the tremendous effort to refine and expand this therapy. We can expect CAR-T to become safer and even more effective with time as these studies bear fruit.
Conclusion
CAR-T cell therapy represents a remarkable marriage of modern science and medicine – leveraging the body’s own immune system, enhanced by genetic engineering, to fight cancer. It has already changed the lives of many patients who had no other hope, offering remission and even cures in certain dire cases. As with any breakthrough, there have been challenges in making it widely available, from managing side effects to the high cost and complex manufacturing process.
Today, we stand at a hopeful turning point. Initiatives like the one by Burjeel Hospital, Abu Dhabi in the UAE are breaking down the barriers of cost and access, embodying the principle that medical innovations should benefit all people, not just a few. By combining global expertise with local action, such programs are bringing cutting-edge treatments closer to patients who need them most.
For patients and families facing cancers like leukemia or lymphoma, CAR-T therapy offers a new beacon of hope. If you or someone you know is confronting a cancer diagnosis, it’s worth discussing with your healthcare team whether CAR-T cell therapy is an option, either as part of standard care or through clinical trials. Thanks to growing experience and expanding programs worldwide, this once-experimental therapy is rapidly becoming part of the standard arsenal in cancer care – an inspiring example of how innovation, collaboration, and commitment to patient welfare can revolutionize what’s possible in medicine.

